EDC Migrator Overview
EDC Migrator helps clinical data teams move clinical data originally sourced from platforms like Medidata RAVE® and Oracle InForm®, directly into Veeva Clinical Data.
The tool is useful for organizations transitioning to, or consolidating within, Veeva Clinical Data. The Migrator directly increases efficiency for study changes and improves data quality and the study site experience. By consolidating data, the Migrator also optimizes database performance and reduces costs, as organizations no longer need to pay for licenses to maintain legacy systems.
Veeva Professional Services performs study migrations into Veeva Clinical Data on behalf of the sponsor.
The Migrator only moves execution or instance data, not the study structure itself. You must first design the study in Veeva Studio before the migration process can initiate.
Key Features
The following key features are offered with the EDC Migrator tool:
- Support for data originating from Rave, InForm, and Veeva.
- The ability to import execution or instance data from the source study.
- The ability to import standard output files (CSV) into Veeva Clinical Data and validate against the study design to ensure compliance.
- Verification and quality control on the migrated data, ensuring data accuracy and integrity remain intact.
- No updates. The study migration is a one-time event.
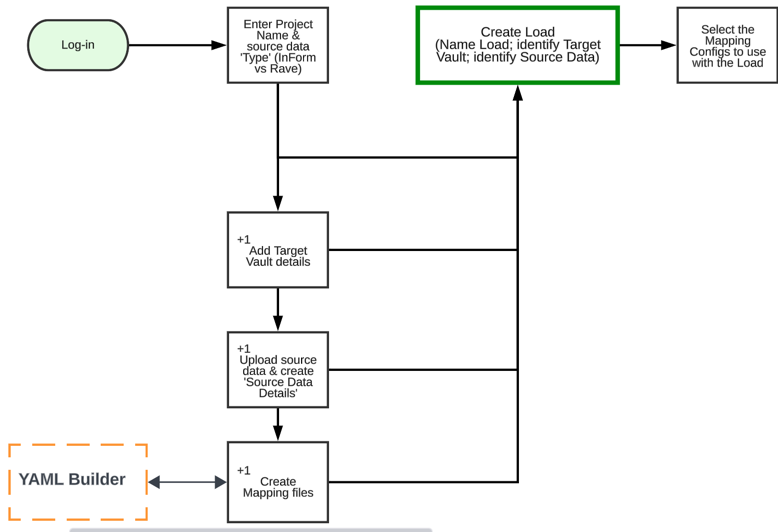
Initial Stages
The workflow below outlines the initial stages of the migration process, including mapping and creating a load.
The Workflow Progress Table provides an overview of all of the migration steps, from creating a load to reconciling listings.